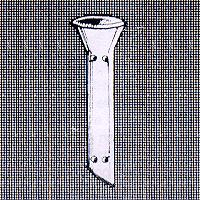
The Pawar intracystic implant has been developed by Dr. M.D. Pawar, an ophthalmic surgeon from Nagpur India, after years of research. Introduction: Pawar. Intracystic implant is a brain child of Dr. M. D. Pawar. Ophthalmic Surgeon from Nagpur, this is a new method for treatment of Epiphora due to obstruction of lacrimal passage e.g. Dacrocystitis. The main aim behind the design is to make treatment of Epiphora simple, quick and effective, by using this implant better, success rates have been obtained. This implant can be used in cases where conventional treatment is contraindicated.
Advantages: Following are important advantages of this implant over conventional treatment: 1. A technically easier and less time consuming procedure. 2. Per operative bleeding is tremendously reduced, no need for nasal packing. 3. Shorter Hospital stay & reduced post operative bleeding. 4. Can be done in all age groups. Infancy is not contraindication. 5. Deformed nasal bridge and senile atrophic mucosa are not complications. 6. Success rates in implant DCR are better than conventional flap dacrocystorhinostomy. 7. Implant in situ does not cause any discomfort or complication. The implant: The Pawar intracystic implant is made of medical grade silicone elastomer, one of the best materials suitable for implantation. The implant has funnel shaped wider end protrudes in the nasal cavity. Following three types of implants are available: 1. Large implant for New Ostium. 2. Large implant for naso lacrimal duct. 3. Small implant for conjunctivo-dacrocysto-rhinostomy operation (when both puncti are blocked). Indications: These are similar to conventional DCR. Contraindications: Same type of contraindications should be considered as in conventional DCR. How supplied: The Pawar intracystic implant is supplied packed in blister peel open packs, ready to use and sterilized by gamma-rays. Each packet contains one implant. Sterilization: The implant should be used once only. However if needed it can be re sterilized by autoclaving. In a clean environment and with gloved hands remove implant from its package. The package is not sterilizable. It should be rinsed with distilled water and then autoclaved by using one of the following methods: 1. High speed autoclave: For 10 minutes at 131 Celsius (2kg/cm^2). 2. Standard autoclave: For 30 minutes at 121 Celsius (2kg/cm^2). Special instruments: For fashioning of new ostium: a. Jenkins type Mastoid gouge 2.5mm or 3mm in size. It can be bought from any surgical dealer. It is a common instrument used in ENT.
b. Blunt lacrimal sac dissector can also be used to create a new ostium. Be careful about the size. c. Special perforator has been developed by surgiwear. It is available in two sizes large and small. For introducing the implant: a. Special introducer has been developed by surgiwear. It is available in two sixes large and small. Operative procedure: Position of patient and anesthesia are similar to as used in conventional DCR. Incision and exposure: Exposure of sac is carried out exactly as that for conventional DCR. Following procedure is suggested for inserting the implant into a new ostium. Incision in the lacrimal sac: A vertical incision around 3mm long is made in the anterior wall of the lacrimal sac. Fashioning the ostium: The ostium is created by using any of the instruments described above, in the lower part of the lacrimal fossa. The instrument passes through the posterior wall of the lacrimal sac, lacrimal bone and nasal mucosa. The instrument points towards posterior, medial and lower direction as shown here. Implantation: A sterilized implant is loaded on the introducer as shown here. Now the implant is introduced through the anterior opening of the lacrimal sac in to the nasal cavity negotiating the posteriomedial wall of the lacrimal sac and newly fashioned ostium. It is placed in such a way that it points towards posterior, medial and lower directions similar to the direction of mastoid gouge. The wider portion (collar) lies in the cavity of the sac and the other end in the middle meatus or lower meatus of the nose. Operative procedure for inserting the implant into the Nasolacrimal duct. Up to the exposure of the lacrimal sac and anterior incision in the wall of the lacrimal sac, the procedure is same. The nasolacrimal duct is dilated by using suitable dilators. The implant is loaded on the introducer and inserted into the dilated naso-lacrimal duct. Position check of the implant: Saline is injected through the funnel of the implant. Observe air bubbles from the nostril via the implant. The position of the implant should be confirmed visually also by inspecting the nostril by using nasal speculum. The pointed portion of the implant should project in the nasal cavity. Closure and patency check after closure: The sac and surgical field is irrigated with normal saline and 1:1000 adrenalin. The wound is closed with 6/0 chromic catgut in layers. The function of implant is may be tested immediately after closure on table itself. The punctum is dilated and syringing is performed. Medication and post operative care: Patient is kept on oral antibiotic and anti inflammatory drugs for 3 days. Topical antibiotic drops are instilled for the period of one month. De congestive nasal drops are used in the nostril of operated side four to six times in a day for one week. First syringing is done on third day and repeated once a week for four weeks. Complications: The main post operative complications are blockage of implant and infection. Blockage in immediate post operative phase is mostly due to clot or improper insertion of imlant through the mucosa. Sometimes the implant does not pass through the nasal mucosa and nasal tenting occurs. On table tests may show correct procedure but as the mucosa heals the implant is blocked. During operation bleeding points should be taken care of. Wound, during operation, may be irrigated with 1:1000 adrenalin. Late blockage of nasal lacrimal passage via implant is due to infection and granulation tissue formation in 2% of the cases. Persistent infection may warrant removal of device. Ordering information: Can be ordered directly or through local dealer. Precautions: Products made of silicone elastomer should not come in contact with lint, glove talc, oily residue from skin, oil based soaps, synthetic detergents or other surface contaminants. Use only thick sutures/ligatures for securing silicone products. Silicone has very poor cut strength. Package should be opened only in clean and controlled environment. Avoid unnecessary handing. Instruments coming in direct contact with silicone products should have soft covers. Warranty: Surgiwear warrants that device has been manufactured with best quality raw material and reasonable care has been taken in manufacturing of this device. Surgiwear will not be liable for any incidental or consequential loss, damage or expense directly and indirectly arising from use of this device. The liability of Surgiwear is limited to the replacement of the product should. Surgiwear's investigation show that the product was defective at the time of its' shipment. No person has any authority to bind Surgiwear to any representation of warranty concerning this device. The information given in this brochure is not exhaustive. It is meant for broad guidance only. The surgeon is advised to use the method which his own practice and discretion dictate to be best for the patient. Returned goods policy: Surgiwear will accept this product for replacement or credit. Provided it is returned in unopened and unsoiled packages, unless returned due to a complaint of product or mislabelling. Products will not be accepted for replacement or credit, if they have been in possession of customer for more than 90 days. Determination of product defect and mislabelling will be made by Surgiwear and will be final.
Advantages: Following are important advantages of this implant over conventional treatment: 1. A technically easier and less time consuming procedure. 2. Per operative bleeding is tremendously reduced, no need for nasal packing. 3. Shorter Hospital stay & reduced post operative bleeding. 4. Can be done in all age groups. Infancy is not contraindication. 5. Deformed nasal bridge and senile atrophic mucosa are not complications. 6. Success rates in implant DCR are better than conventional flap dacrocystorhinostomy. 7. Implant in situ does not cause any discomfort or complication. The implant: The Pawar intracystic implant is made of medical grade silicone elastomer, one of the best materials suitable for implantation. The implant has funnel shaped wider end protrudes in the nasal cavity. Following three types of implants are available: 1. Large implant for New Ostium. 2. Large implant for naso lacrimal duct. 3. Small implant for conjunctivo-dacrocysto-rhinostomy operation (when both puncti are blocked). Indications: These are similar to conventional DCR. Contraindications: Same type of contraindications should be considered as in conventional DCR. How supplied: The Pawar intracystic implant is supplied packed in blister peel open packs, ready to use and sterilized by gamma-rays. Each packet contains one implant. Sterilization: The implant should be used once only. However if needed it can be re sterilized by autoclaving. In a clean environment and with gloved hands remove implant from its package. The package is not sterilizable. It should be rinsed with distilled water and then autoclaved by using one of the following methods: 1. High speed autoclave: For 10 minutes at 131 Celsius (2kg/cm^2). 2. Standard autoclave: For 30 minutes at 121 Celsius (2kg/cm^2). Special instruments: For fashioning of new ostium: a. Jenkins type Mastoid gouge 2.5mm or 3mm in size. It can be bought from any surgical dealer. It is a common instrument used in ENT.
b. Blunt lacrimal sac dissector can also be used to create a new ostium. Be careful about the size. c. Special perforator has been developed by surgiwear. It is available in two sizes large and small. For introducing the implant: a. Special introducer has been developed by surgiwear. It is available in two sixes large and small. Operative procedure: Position of patient and anesthesia are similar to as used in conventional DCR. Incision and exposure: Exposure of sac is carried out exactly as that for conventional DCR. Following procedure is suggested for inserting the implant into a new ostium. Incision in the lacrimal sac: A vertical incision around 3mm long is made in the anterior wall of the lacrimal sac. Fashioning the ostium: The ostium is created by using any of the instruments described above, in the lower part of the lacrimal fossa. The instrument passes through the posterior wall of the lacrimal sac, lacrimal bone and nasal mucosa. The instrument points towards posterior, medial and lower direction as shown here. Implantation: A sterilized implant is loaded on the introducer as shown here. Now the implant is introduced through the anterior opening of the lacrimal sac in to the nasal cavity negotiating the posteriomedial wall of the lacrimal sac and newly fashioned ostium. It is placed in such a way that it points towards posterior, medial and lower directions similar to the direction of mastoid gouge. The wider portion (collar) lies in the cavity of the sac and the other end in the middle meatus or lower meatus of the nose. Operative procedure for inserting the implant into the Nasolacrimal duct. Up to the exposure of the lacrimal sac and anterior incision in the wall of the lacrimal sac, the procedure is same. The nasolacrimal duct is dilated by using suitable dilators. The implant is loaded on the introducer and inserted into the dilated naso-lacrimal duct. Position check of the implant: Saline is injected through the funnel of the implant. Observe air bubbles from the nostril via the implant. The position of the implant should be confirmed visually also by inspecting the nostril by using nasal speculum. The pointed portion of the implant should project in the nasal cavity. Closure and patency check after closure: The sac and surgical field is irrigated with normal saline and 1:1000 adrenalin. The wound is closed with 6/0 chromic catgut in layers. The function of implant is may be tested immediately after closure on table itself. The punctum is dilated and syringing is performed. Medication and post operative care: Patient is kept on oral antibiotic and anti inflammatory drugs for 3 days. Topical antibiotic drops are instilled for the period of one month. De congestive nasal drops are used in the nostril of operated side four to six times in a day for one week. First syringing is done on third day and repeated once a week for four weeks. Complications: The main post operative complications are blockage of implant and infection. Blockage in immediate post operative phase is mostly due to clot or improper insertion of imlant through the mucosa. Sometimes the implant does not pass through the nasal mucosa and nasal tenting occurs. On table tests may show correct procedure but as the mucosa heals the implant is blocked. During operation bleeding points should be taken care of. Wound, during operation, may be irrigated with 1:1000 adrenalin. Late blockage of nasal lacrimal passage via implant is due to infection and granulation tissue formation in 2% of the cases. Persistent infection may warrant removal of device. Ordering information: Can be ordered directly or through local dealer. Precautions: Products made of silicone elastomer should not come in contact with lint, glove talc, oily residue from skin, oil based soaps, synthetic detergents or other surface contaminants. Use only thick sutures/ligatures for securing silicone products. Silicone has very poor cut strength. Package should be opened only in clean and controlled environment. Avoid unnecessary handing. Instruments coming in direct contact with silicone products should have soft covers. Warranty: Surgiwear warrants that device has been manufactured with best quality raw material and reasonable care has been taken in manufacturing of this device. Surgiwear will not be liable for any incidental or consequential loss, damage or expense directly and indirectly arising from use of this device. The liability of Surgiwear is limited to the replacement of the product should. Surgiwear's investigation show that the product was defective at the time of its' shipment. No person has any authority to bind Surgiwear to any representation of warranty concerning this device. The information given in this brochure is not exhaustive. It is meant for broad guidance only. The surgeon is advised to use the method which his own practice and discretion dictate to be best for the patient. Returned goods policy: Surgiwear will accept this product for replacement or credit. Provided it is returned in unopened and unsoiled packages, unless returned due to a complaint of product or mislabelling. Products will not be accepted for replacement or credit, if they have been in possession of customer for more than 90 days. Determination of product defect and mislabelling will be made by Surgiwear and will be final.
Specifications
- Bibliography:
- Dejean: Arch Ophthalmol 51:102,1954.
- Eriysher PA: Ophthalm Lit 15:1309 1961.
- Msione M: Ibid 7:5142 1953.
- Pentini G. Florini G: Ibid 13:4950 1959.
- Roif & Jackson (1952) quoted in Duke-Elder's system of Ophthalmology. Vol Xll part 2, page 715 London. Henry Kimpton, 1974.
- Zeeper R: Amer. J. Ophthalmology 63: 1837, 1967.
- Summerskill (1952), Quoted by Duke Elder's system of Ophthalmology Vol. Xll part 2, page 715 London, Henry Kimpton,1974.
- Pawar MD and Patil SN: Acta XXV Concilium Ophthalmologicum pp. 1988-1992, 1987.
Main Products
silicone implantable devices, disposable drapes & dressings calcium hydroxyapatite (g-bone), (G-eye), fallopian tubal ring

