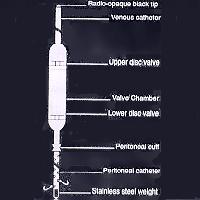
Gisaims I: a. The proximal/peritoneal catheter: It is a 40cm long tube of which the proximal 30cm is perforated. There is a cuff at the junction of the perforated and plain segments for a purse string suture. b. Main valve body: This is made of silicone elastomer. It has two disc valves to facilitate the pumping action. The valves are unidirectional and allow flow of fluid at a 3-cm water pressure gradient. The valve chamber is placed on the rid cage so that its middle portion can be depressed to pump the peritoneal fluid upwards. c. Venous catheter: This is a plain tube with a radio-opaque distal end. Its tip should lie just proximal to the right atrium.
Gisaims II for adults & Gisaims III for children. Single valve types: Here the only difference is that the main valve chamber has a single valve which is placed in the anterior abdominal wall during implantation. The Gisaims peritoneo-venous shunt has been developed by Surgiwear after six years of research in collaboration with some of the most prominent Gastro-intestiral surgeons in India. Introduction: The peritoneo-venous shunt has a limited but definite place in the treatment of a patient who has ascites which is resistant to medical management. It reduces abdominal girth and weight, improves plasma volume and is associated with an improvement of renal function and a reduction of salt retention. In contrast medical therapy seems to compromise renal function. Although the peritoneo-venous shunt does not prolong life, it does improve its quality. There are complications associated with its use and it should, therefore, be inserted with care. The mechanism of action of the Gisaims shunt: The ascitic fluid enters the peritoneal catheter, forces open the valve and flows towards the right atrium via the distal catheter which discharge the fluid into the venous circulation. In the double valve type of shunt depression of the middle of the valve body forces the fluid towards the atrium. This action closes the proximal valve preventing reverse flow and when the pressure is removed, the valve body re-inflates, with closure of the distal valve and opening of the proximal valve. How supplied: Gisaims peritoneo-venous shunts are supplied as complete unitized system, sterilized by gamma-rays and ready to use in double peel open packaging. Each packet contains one main valve body with proximal and distal catheter in unitized form and two connectors. Each component is also available in separate packaging. Materials: The materials used in Gisaims peritoneo-venous shunts are medical grade silicone elastomer, stainless steel, polypropylene and tantalum powder. Indication: Failure of adequate conservative treatment for 3 weeks, i.e. low salt intake and diuretic therapy (160mg frusemide and 400mg spironolactone) is the main indication for shunt placement. Other patients who cannot afford and do not comply with these massive diuretic doses may do better with peritoneo-venous shunts. The shunt should be placed before the development of hepatic and renal failure in the following conditions: Malignant ascites, budd-chiari syndrome, chylous ascites, nephrogenic ascites, biliary atresia, amyloidosis, cryptogenic ascites, hepatorenal syndrome, hydrothorax secondary to ascites. Contraindications: These include peritonitis, recent variceal bleeding, cardiac dysfunction and acute tubular necrosis. Preoperative preparation: Base line investigations, full blood picture, serum electrolytes, liver function tests, serum creatinine, coagulation tests, daily urine output, weight and abdominal girth should be done. To reduce the risk of intravascular coagulation, some surgeons drain most of the ascitic fluid before surgery. Cleaning and autoclaving: The Gisaims shunt as supplied is sterile. It may also be re-sterilized. In a clean environment and with gloved hands remove shunt from its package. The packaging is not sterilizable. It should be rinsed and flushed with sterile distilled water, It may be wrapped in lint free material and then autoclaved for 30 minutes at 121 Celsius and 1kg/cm pressure. Ethylene oxide sterilization is not recommended. Preparation of the device: Some surgeons recommend that the shunt should be immersed in antibiotic solution (80mg gentamicin sulphate in 500ml normal saline) and the chamber pumped (Gisaims I) till the tubes are filled with this solution and flow established. All air bubbles should be expelled by elevating the venous end of the shunt and tapping the valve and tubes. Operative procedure: The operation is performed using either local or general anesthesia with the patient in the supine position and the neck slightly extended: A. Valve placement: Gisaims I: A 5cm transverse incision is made below the right costal margin in the anterior auxillary line and carried down to the muscle. A space is fashioned over the lower chest wall using the index finger to allow placement of the pump chamber. The external and internal oblique muscles are split down to the transversalis fascia and two concentric purse string sutures of 3-0 silk placed (1.5cm and 2.0cm diameter). Gisaims II: The transverse incision is placed at the level of the umbilicus in the anterior axillary line. The concentric purse string sutures are placed in the transversalis fascia and the space for the valve created deep to the muscle layers. B. Neck incision: A 3cm collar incision is made in the right side of the neck extending from the midline to the sternomastoid muscles. The internal jugular vein is isolated and looped with 2-0 silk ligatures. C. Insertion into the peritoneal cavity: A small hole is made in the peritoneum at the center of the previously placed purse string sutures and the peritoneal catheter inserted so that its tip lies in the pelvis. The sutures are then tied over the cuff to obtain a water tight closure. After confirming that there is free low of ascitic fluid through the venous end the tube is clamped (the clamp should have soft rubber covers). D. Tunneling: A long introducer (obtainable from surgiwear) is pushed subcutaneously from the abdominal wound into the neck and a No. 1 silk suture pulled downwards. The tip of the venous tubing is fixed to this suture and pulled up into the neck. The abdominal incision is now carefully closed in layers. E. Insertion of venous catheter into the jugular vein: With the patient in the trendelenburg position (to minimize the risk of an air embolus) the venous catheter is passed in the internal jugular vein so that its tip lies at the level of the second intercostal space (this should be cut off near the valve body and a connector used to join the cut ends). So that it makes a smooth curve. The clamp should be removed and ascitic fluid should be allowed to flow out freely before insertion of the tube into the jugular vein. The neck incision is then closed. Postoperative management: The patient is placed in a sitting (45 degree head up ) position and given 40mg frusemide intravenous injection immediately. The urine output is measured hourly, coagulation studies are performed daily and the abdominal girth, body weight, serum electrolytes and liver function tests are measured three times a week. Complications: 1. Shunt blockage: This occurs more often if the details of the surgical technique are not followed. Patency can be tested in the Gisaims I by depressing the pump chamber. Resistance to compression indicates that the venous end is blocked. If after compression the pump body does not refill this indicates obstruction of the peritoneal tube. The patency of the Gisaims II type shunt can be determined by a doppler flow meter, by injecting Technetium Sulphur colloid into the peritoneal cavity or contrast media into the venous tubing using a 26 G needle directed obliquely. 2. Dsseminated intravascular coagulation: This is probably due to the presence of pro coagulants in the ascitic fluid and its incidence is minimized if most of the ascitic fluid is discarded at the time of shunt placement. 3. Infection: This may occur due to leakage of ascitic fluid around the peritoneal catheter and along the shunt and may necessitate its removal. 4. Fever: Low grade fever occurs during the early postoperative period but is self-limiting. 5. Heart failure: Large amounts of ascitic fluid enter the blood stream through a functioning peritoneo-venous shunt. This can overload and already impaired heart, this complication can be prevented preoperatively by draining the ascitic fluid before the shunt insertion in patients with cardiac disease. Returned goods policy: Surgiwear will accept this product for replacement or credit. Provided it is returned in unopened and unsoiled packages, unless returned due to a complaint of product or mislabelling. Products will not be accepted for replacement or credit, if they have been in possession of customer for more than 90 days. Determination of product defect and mislabelling will be made by Surgiwear and will be final. Precautions: Products made of silicone elastomer should not come in contact with lint, glove talc, oily residue from skin, oil based soaps, synthetic detergents or other surface contaminants. Use only thick sutures / ligatures for securing silicone products. Silicone has very poor cut strength. Package should be opened only in clean and controlled environment. Avoid unnecessary handing. Instruments coming in direct contact with silicone products should have soft covers. The information given in this brochure is not exhaustive. It is meant for broad guidance only. The surgeon is advised to use the method which his own practice and discretion dictate to be best for the patient. Warranty: Surgiwear warrants that device has been manufactured with best quality raw material and reasonable care has been taken in manufacturing of this device. Surgiwear will not be liable for any incidental or consequential loss, damage or expense directly and indirectly arising from use of this device. The liability of Surgiwear is limited to the replacement of the product should. Surgiwear's investigation show that the product was defective at the time of its' shipment. No person has any authority to bind Surgiwear to any representation of warranty concerning this device.
Gisaims II for adults & Gisaims III for children. Single valve types: Here the only difference is that the main valve chamber has a single valve which is placed in the anterior abdominal wall during implantation. The Gisaims peritoneo-venous shunt has been developed by Surgiwear after six years of research in collaboration with some of the most prominent Gastro-intestiral surgeons in India. Introduction: The peritoneo-venous shunt has a limited but definite place in the treatment of a patient who has ascites which is resistant to medical management. It reduces abdominal girth and weight, improves plasma volume and is associated with an improvement of renal function and a reduction of salt retention. In contrast medical therapy seems to compromise renal function. Although the peritoneo-venous shunt does not prolong life, it does improve its quality. There are complications associated with its use and it should, therefore, be inserted with care. The mechanism of action of the Gisaims shunt: The ascitic fluid enters the peritoneal catheter, forces open the valve and flows towards the right atrium via the distal catheter which discharge the fluid into the venous circulation. In the double valve type of shunt depression of the middle of the valve body forces the fluid towards the atrium. This action closes the proximal valve preventing reverse flow and when the pressure is removed, the valve body re-inflates, with closure of the distal valve and opening of the proximal valve. How supplied: Gisaims peritoneo-venous shunts are supplied as complete unitized system, sterilized by gamma-rays and ready to use in double peel open packaging. Each packet contains one main valve body with proximal and distal catheter in unitized form and two connectors. Each component is also available in separate packaging. Materials: The materials used in Gisaims peritoneo-venous shunts are medical grade silicone elastomer, stainless steel, polypropylene and tantalum powder. Indication: Failure of adequate conservative treatment for 3 weeks, i.e. low salt intake and diuretic therapy (160mg frusemide and 400mg spironolactone) is the main indication for shunt placement. Other patients who cannot afford and do not comply with these massive diuretic doses may do better with peritoneo-venous shunts. The shunt should be placed before the development of hepatic and renal failure in the following conditions: Malignant ascites, budd-chiari syndrome, chylous ascites, nephrogenic ascites, biliary atresia, amyloidosis, cryptogenic ascites, hepatorenal syndrome, hydrothorax secondary to ascites. Contraindications: These include peritonitis, recent variceal bleeding, cardiac dysfunction and acute tubular necrosis. Preoperative preparation: Base line investigations, full blood picture, serum electrolytes, liver function tests, serum creatinine, coagulation tests, daily urine output, weight and abdominal girth should be done. To reduce the risk of intravascular coagulation, some surgeons drain most of the ascitic fluid before surgery. Cleaning and autoclaving: The Gisaims shunt as supplied is sterile. It may also be re-sterilized. In a clean environment and with gloved hands remove shunt from its package. The packaging is not sterilizable. It should be rinsed and flushed with sterile distilled water, It may be wrapped in lint free material and then autoclaved for 30 minutes at 121 Celsius and 1kg/cm pressure. Ethylene oxide sterilization is not recommended. Preparation of the device: Some surgeons recommend that the shunt should be immersed in antibiotic solution (80mg gentamicin sulphate in 500ml normal saline) and the chamber pumped (Gisaims I) till the tubes are filled with this solution and flow established. All air bubbles should be expelled by elevating the venous end of the shunt and tapping the valve and tubes. Operative procedure: The operation is performed using either local or general anesthesia with the patient in the supine position and the neck slightly extended: A. Valve placement: Gisaims I: A 5cm transverse incision is made below the right costal margin in the anterior auxillary line and carried down to the muscle. A space is fashioned over the lower chest wall using the index finger to allow placement of the pump chamber. The external and internal oblique muscles are split down to the transversalis fascia and two concentric purse string sutures of 3-0 silk placed (1.5cm and 2.0cm diameter). Gisaims II: The transverse incision is placed at the level of the umbilicus in the anterior axillary line. The concentric purse string sutures are placed in the transversalis fascia and the space for the valve created deep to the muscle layers. B. Neck incision: A 3cm collar incision is made in the right side of the neck extending from the midline to the sternomastoid muscles. The internal jugular vein is isolated and looped with 2-0 silk ligatures. C. Insertion into the peritoneal cavity: A small hole is made in the peritoneum at the center of the previously placed purse string sutures and the peritoneal catheter inserted so that its tip lies in the pelvis. The sutures are then tied over the cuff to obtain a water tight closure. After confirming that there is free low of ascitic fluid through the venous end the tube is clamped (the clamp should have soft rubber covers). D. Tunneling: A long introducer (obtainable from surgiwear) is pushed subcutaneously from the abdominal wound into the neck and a No. 1 silk suture pulled downwards. The tip of the venous tubing is fixed to this suture and pulled up into the neck. The abdominal incision is now carefully closed in layers. E. Insertion of venous catheter into the jugular vein: With the patient in the trendelenburg position (to minimize the risk of an air embolus) the venous catheter is passed in the internal jugular vein so that its tip lies at the level of the second intercostal space (this should be cut off near the valve body and a connector used to join the cut ends). So that it makes a smooth curve. The clamp should be removed and ascitic fluid should be allowed to flow out freely before insertion of the tube into the jugular vein. The neck incision is then closed. Postoperative management: The patient is placed in a sitting (45 degree head up ) position and given 40mg frusemide intravenous injection immediately. The urine output is measured hourly, coagulation studies are performed daily and the abdominal girth, body weight, serum electrolytes and liver function tests are measured three times a week. Complications: 1. Shunt blockage: This occurs more often if the details of the surgical technique are not followed. Patency can be tested in the Gisaims I by depressing the pump chamber. Resistance to compression indicates that the venous end is blocked. If after compression the pump body does not refill this indicates obstruction of the peritoneal tube. The patency of the Gisaims II type shunt can be determined by a doppler flow meter, by injecting Technetium Sulphur colloid into the peritoneal cavity or contrast media into the venous tubing using a 26 G needle directed obliquely. 2. Dsseminated intravascular coagulation: This is probably due to the presence of pro coagulants in the ascitic fluid and its incidence is minimized if most of the ascitic fluid is discarded at the time of shunt placement. 3. Infection: This may occur due to leakage of ascitic fluid around the peritoneal catheter and along the shunt and may necessitate its removal. 4. Fever: Low grade fever occurs during the early postoperative period but is self-limiting. 5. Heart failure: Large amounts of ascitic fluid enter the blood stream through a functioning peritoneo-venous shunt. This can overload and already impaired heart, this complication can be prevented preoperatively by draining the ascitic fluid before the shunt insertion in patients with cardiac disease. Returned goods policy: Surgiwear will accept this product for replacement or credit. Provided it is returned in unopened and unsoiled packages, unless returned due to a complaint of product or mislabelling. Products will not be accepted for replacement or credit, if they have been in possession of customer for more than 90 days. Determination of product defect and mislabelling will be made by Surgiwear and will be final. Precautions: Products made of silicone elastomer should not come in contact with lint, glove talc, oily residue from skin, oil based soaps, synthetic detergents or other surface contaminants. Use only thick sutures / ligatures for securing silicone products. Silicone has very poor cut strength. Package should be opened only in clean and controlled environment. Avoid unnecessary handing. Instruments coming in direct contact with silicone products should have soft covers. The information given in this brochure is not exhaustive. It is meant for broad guidance only. The surgeon is advised to use the method which his own practice and discretion dictate to be best for the patient. Warranty: Surgiwear warrants that device has been manufactured with best quality raw material and reasonable care has been taken in manufacturing of this device. Surgiwear will not be liable for any incidental or consequential loss, damage or expense directly and indirectly arising from use of this device. The liability of Surgiwear is limited to the replacement of the product should. Surgiwear's investigation show that the product was defective at the time of its' shipment. No person has any authority to bind Surgiwear to any representation of warranty concerning this device.
Main Products
silicone implantable devices, disposable drapes & dressings calcium hydroxyapatite (g-bone), (G-eye), fallopian tubal ring

