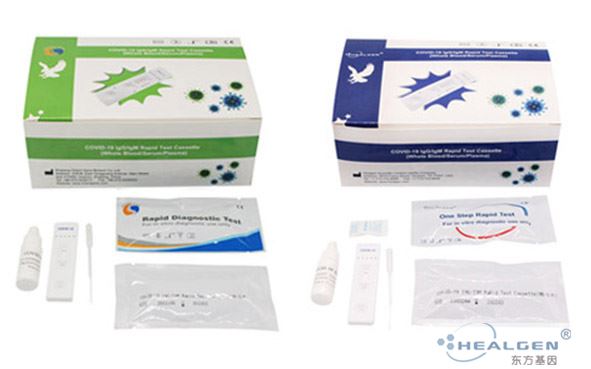
The COVID-19 IgG/IgM (Whole Blood/Serum/Plasma) Rapid Test Device utilizes lateral flow technology for the qualitative, differential detection of both anti-SARS-CoV-2 IgM and IgG antibodies.
In general, antibodies can be detected 1-3 weeks after infection. This test is intended to screen patients for SARS-CoV-2 antibodies
Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals and birds that cause respiratory, enteric, hepatic and neurologic diseases. Four viruses - 229E, OC43, NL63 and HKU1 are prevalent and typically cause common cold symptoms in immunocompromised individuals. Three other strains SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) can be transmitted from between non-human vertebrates to humans.
In general, antibodies can be detected 1-3 weeks after infection. This test is intended to screen patients for SARS-CoV-2 antibodies
Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals and birds that cause respiratory, enteric, hepatic and neurologic diseases. Four viruses - 229E, OC43, NL63 and HKU1 are prevalent and typically cause common cold symptoms in immunocompromised individuals. Three other strains SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) can be transmitted from between non-human vertebrates to humans.
Certificate
- CE/FDA
Main Products
Colloidal gold rapid diagnostic test/Dry chemical strips/Quantitative
